Clean room technology is a common sight in industry. The technology and air quality involved in the production, processing and assembly of electronics, precision engineering and optics, as well as in the pharmaceutical, biotechnology and food manufacturing industries, is subject to incredibly high standards. The only way to reliably manufacture products of a consistently high quality and, often, to protect employees' health is by ensuring that the air in the workplace complies with defined air quality standards.
These processes often involve flammable substances in the form of liquids or dust particles. When all systems are running smoothly, these would of course be contained within closed equipment. In the event of a fault, however, these substances may be released, creating an explosive atmosphere. Explosion hazards also occur when equipment is being cleaned with flammable solvents. This is why, even in clean rooms, it is important that the explosion protection requirements are fulfilled. This article will describe how this is done.
Overview of clean room technology
Generally speaking, clean room technology is designed to protect specific work areas from unwanted external influencing factors. These external influences occur in the form of contaminants, which can be gaseous, or liquid or solid particles of various sizes. These particles can have a harmful effect in a number of ways:
- The trend towards miniaturisation, which is most noticeable in microelectronics and microstructure technology, has meant that even the smallest quantities of tiny particles that are not part of the process can lead to long-lasting adverse effects on product quality. This fact becomes more understandable if we consider the typical sizes of structures in microelectronics nowadays, which are often in the sub-micrometre range, and then compare these dimensions with the average size of a particle of household dust, orders of magnitude larger.
- The manufacture of many pharmaceutical products requires a high degree of asepsis. Germs, dust and, in particular, microorganisms have an adverse effect on the quality of pharmaceutical compounds and can, under the right circumstances, also cause side effects with a serious health risk.
- Many active substances used in the pharmaceutical industry are highly active and toxic. Even just coming into contact with a tiny amount of these substances can cause severe health issues for employees in production.
In general, clean room technology has two aims. Firstly, to protect products, equipment and production processes from damage caused by contamination and from output losses, and secondly, to protect the people involved in the production processes against any process-related health risks. This is done by ensuring that the work environment has a precisely specified air purity in relation to airborne particulate foreign matter.
When designing and setting up clean rooms, the end user and the system set-up engineer must work closely together to ensure that the requisite cleanliness standards are achieved at a reasonable cost. The implemented solution must be optimally tailored to the application for which it is intended. Important input variables in this process include a range of process parameters, such as the number of people active at any point, the necessity of protective equipment, heat build-up, the substances to be processed, the required cleanliness set out in cleanliness classes, the installation density, and the building structure. Often, there are also a number of official regulations that need to be complied with.
The close link between the design of a clean room and its intended purpose may be one reason why the landscape of international and national standards and directives regarding clean rooms was very heterogeneous and fragmented for a long time. It was not until a few years ago that this field saw a tangible development, which enabled manufacturers of equipment for clean rooms to supply standardised products for a range of applications.
In order to guarantee safe batch production in subsequent operation, it must be ensured that all critical clean room parameters, including the temperature, humidity, pressure difference and particle count in particular, are continuously recorded and documented. Based on increasingly strict requirements regarding the ambient conditions for the manufacture and filling of medicinal products set out by international and national authorities such as the WHO, the EU or the American FDA, continuous monitoring of the ambient conditions as well as complete documentation of the corresponding recorded data has become an absolute imperative.
Explosion protection in clean rooms
The substances and active ingredients processed in clean rooms are very often combustible in both dust and liquid form; as a result, they can form a hazardous explosive atmosphere together with the oxygen in the air. Although most processes take place in closed equipment due to the particular requirements for the ambient conditions, there may also be process steps such as filling particulate substances or liquids, in which combustible substances are handled in small quantities outside closed containers. Furthermore, when determining the explosion risk, it is important to consider a range of system statuses in which clean room conditions are not maintained and an explosion hazard may be present. System statuses of this kind include test runs, starting up a system, test processes and maintenance work.
Cleaning processes are a special case. Often, solvents must be used to thoroughly clean the rooms and equipment. This requires classification in a hazardous area, even if no combustible substances are used in the process.
The classification of the hazardous clean rooms themselves is based on the type of combustible substances used and the frequency of occurrence of an explosive atmosphere.
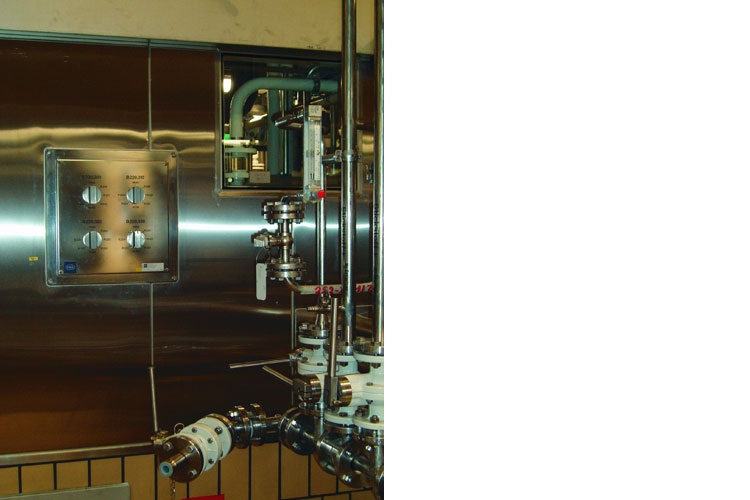
Example of a clean room system with hazardous areas
The following section will explain the explosion protection measures in clean rooms using a system belonging to a large pharmaceutical manufacturer as an example. The strict cleanliness requirements are process-related as well as being required due to the presence of some highly reactive substances.
The system was planned within three years and set up over several floors in an existing building. Since combustible solvents are used regularly to clean the rooms, the required explosion protection measures needed to be implemented.
The reactor rooms are located on the top floor. This term is somewhat misleading, as the large reactors are installed vertically over several floors of the building – only the upper areas of the reactors are located in these rooms. Because the processes are predominantly closed, there are no specific requirements with regard to the maximum permitted concentration of particulate matter in these rooms. However, there are requirements covering the cleaning of the rooms and the equipment within. In order to protect people, contaminants must be easy to remove completely.
The automation technology in the system is based on networked remote PCs, which have no connection to a superordinate distributed control system, and a fieldbus system (PROFIBUS PA) that is suitable for use in Zone 1 and that networks sensors and actuators that are capable of communication. The processes that take place in each room can be directly controlled from the remote PCs. Remote control from a different room is not possible – only safety-critical commands can be transmitted to another PC.
The mixing room is on the floor below. This is where the substances are premixed – paste substances are produced by mixing various starting materials together using solvents. Strict process cleanliness requirements apply here as the starting materials are mainly handled in the open – contamination is restricted to a maximum of 100,000 particles per cubic foot.
All walls are made from stainless steel. They are designed such that no dust deposits can accumulate and they are easy to clean. The floor has been ground and painted multiple times. Explosion-protected stainless steel ceiling-mounted lights in device category 2 provide lighting. Due to the specific work requirements, a high illuminance is required. To protect the staff, both the mixing room and reactor room must only be entered via an air lock by people wearing an externally ventilated protective suit. The aforementioned ceiling-mounted lights are also installed here.
It is important that the movement of people and the transportation of materials are kept strictly separate from one another throughout the entire system. For this reason, in addition to the air locks for personnel, there are separate air locks for materials.
The bottom section of the reactor is on the ground floor. This is where the reaction products are removed and processed further using centrifuges and different drying equipment.
In the basement, there are various vessels that serve to collect wastewater and perform safety functions. In the event of an emergency system shutdown, all of the reactor liquid is collected in one vessel. There are no particular purity requirements here; however, due to the risk of hazardous explosive atmospheres being created, explosion-protected equipment, such as linear luminaires (Zone 2), safety switches and operating terminals (Zone 1), is provided.
Summary
The combination of clean room technology and explosion protection represents a confluence of two typically niche sectors, the importance of which is down to the unique requirements of certain industrial processes. While clean room technology is responsible for protecting products and people, explosion protection takes on the task of protecting people and systems from the hazardous effects of uncontrolled process reactions. The technical and organisational requirements for clean room technology and explosion protection are fundamentally different.
To avoid unnecessarily high costs when setting up systems that require both types of protection, close collaboration between the planner, system user, and explosion-protected equipment manufacturer is essential from an early stage.












![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/user_upload/mitarbeiter/01_DE/07_Blog/00_Allgemein/blog-explosionsschutz-rstahl-startseite-279x205.jpg)
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/user_upload/mitarbeiter/01_DE/07_Blog/00_Allgemein/blog-explosionsschutz-rstahl-ueber-den-blog-279x205.jpg)
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/user_upload/mitarbeiter/01_DE/07_Blog/00_Allgemein/blog-explosionsschutz-rstahl-autoren-279x205.jpg)
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/user_upload/mitarbeiter/01_DE/07_Blog/00_Allgemein/blog-explosionsschutz-rstahl-newsletter-expert-mail-279x205.jpg)
Write new comment